It’s Just…So…Cool – Don’t miss your opportunity for JSC!
By Kim Joline Schmidt, MSc, Mareike Konstanski, MSc, Erika Wissinger, PhD, Patrick Blank, PhD
The implementing acts on Joint Scientific Consultation (JSC) and Joint Clinical Assessment (JCA) by the European Commission (EC) are eagerly awaited by the industry and national health technology assessment (HTA) authorities. What are current developments, and how can stakeholders prepare for the European Union (EU) HTA during the interim period?

While we are several months into the interim period between the closure of EUnetHTA 21 and the full application of the HTA Regulation (HTAR), uncertainties still remain on some aspects of the new EU HTA procedure starting in January 2025. Biopharma and medical device companies are still awaiting the implementing acts on the JCA and JSC by the EC for final guidance on the development of JSC briefing documents and JCA submission dossiers (see Table 1 for timelines of implementing acts). All stakeholders eagerly await the public consultation. In addition, more information on the national implementation of the EU HTA is outstanding, such as how current local HTA procedures are anticipated to change, which additional data analyses may be requested for the national dossiers building on top of the JCA report (so-called delta dossiers), and what impact this may have on timelines and patient access on a country level.
Table 1. Implementing acts to be adopted by 2025| Implementing act | Deadline |
|---|---|
| Procedural rules for JCA medicinal products | Published for public consultation here |
| Procedural rules for the prevention of conflict of interest | Q2-Q3 2024 |
| Cooperation by exchange of information with the EMA | Q2-Q3 2024 |
| Procedural rules for JSC medicinal products | Q2-Q3 2024 |
| Procedural rules for JCA medical devices and IVD medical devices |
Q4 2024 |
| Procedural rules for JSC medical devices and IVD medical devices | Q4 2024 |
Key: EMA – European Medicines Agency; IVD – in vitro diagnostic; JCA – Joint Clinical Assessment; JSC – Joint Scientific Consultation.
Source: European Commission. Implementation rolling plan. 2023-2024. Accessed 20 January 2024. https://health.ec.europa.eu/document/download/397b2a2e-1793-48fd-b9f5-7b8f0b05c7dd_en?filename=hta_htar_rolling-plan_en.pdf
Biopharma and medical device companies can use this time until the full implementation of the HTAR efficiently by developing an organizational readiness and JCA submission strategy. In parallel, participation in JSCs is a voluntary but integral component of the new EU HTA to get early insights into the evidence requirements needed for the JCA. The Joint Scientific Advice with the European Medicines Agency (EMA) and EU HTA bodies conducted during the interim period until December 2024 and organized by the German Joint Federal Committee (G-BA) is giving preferred consideration to oncology products and/or advanced therapy medicinal products (ATMPs), as only a few slots will be available. However, medical device companies that will be affected by a JCA from 2026 and biopharma companies developing orphan drugs or any other drug falling under the regulation, affected from 2028 and 2030, respectively, should start planning proactively for JSCs beginning next year (from 2025) to inform the setup of their pivotal trials.
What should companies know about the JSC?
Applications for the joint consultations organized by the G-BA during the interim period have been open since September 2023. The first advice meetings are expected to take place in Q2 2024, and will consider a period of 6 months between application and the actual meeting between manufacturers, HTA bodies, and the EMA (see more details on timelines in Figure 1). The EUnetHTA 21 consortium developed timelines and templates for the documents to be submitted (e.g. the application form, the briefing book, and study information) that can be utilized and can be found on the EUnetHTA website and on the website of the G-BA.
During an information event hosted by the G-BA on 10 November 2023, Dr. Stephanie Said, Head of the HTA subgroup “Joint Scientific Consultations” and part of the G-BA, gave more insights into the JSC process starting in 2025 for all indications (and also including medical devices in the second half of 2025). The subgroup is currently working on the final guidance based on the proposed documents from EUnetHTA 21 to be finalized by the end of 2024. As the implementing act for the adoption of procedures related to JSC will likely come into effect by mid-2024, some of the work will have to be done in parallel. Overall, the process will be similar to the parallel EMA/HTA body Scientific Advice. One main difference is that the EMA is not included in the basic model but can be included if requested. This will have an impact on financial aspects, as the sole HTA body advice will be free of charge (as of now), but EMA fees will still apply. In later stages (around 2028), a review is planned to determine whether a fee system should be introduced for the JSC.
Applications for the JSC for medicinal products will be open starting 12 January 2025, during so-called “request periods.” The timing of these still needs to be defined, but a learning from the pilot period from EUnetHTA 21 has been that several request periods throughout the year will be planned. As of now, early advice is only planned to occur once during the process for each specific drug/indication. On top of what the regulation stipulates, questions regarding health economic assessment could also be covered during the JSC.
To be eligible for a JSC, 3 criteria need to be fulfilled:
- The product needs to fall within the scope of the responsibility of the EU HTAR
- The pivotal clinical trial needs to be still in the planning phase
- The health technology is expected to be part of a JCA according to article 7 (1) of the regulation
It is expected that requests for JSC spots will exceed the available spots; in that case, technologies will be selected based on the following selection criteria:
- Unmet medical needs
- First in class
- Potential impact on patients, public health, or healthcare systems
- Significant cross-border dimension
- Major EU-wide added value
- EU clinical research priorities
During the event held by the G-BA in November 2023, it was highlighted several times that this system will need to be a learning and evolving system to be successful in its implementation. There is still some work to be done and less than a year remains to refine the process. The EUnetHTA 21 documents provide a solid basis to build upon for all stakeholders to get familiar with the process in theory and soon also in practice.
Figure 1. Timeline for key milestones in the interim EMA/HTA body Scientific Advice procedure (in working days)
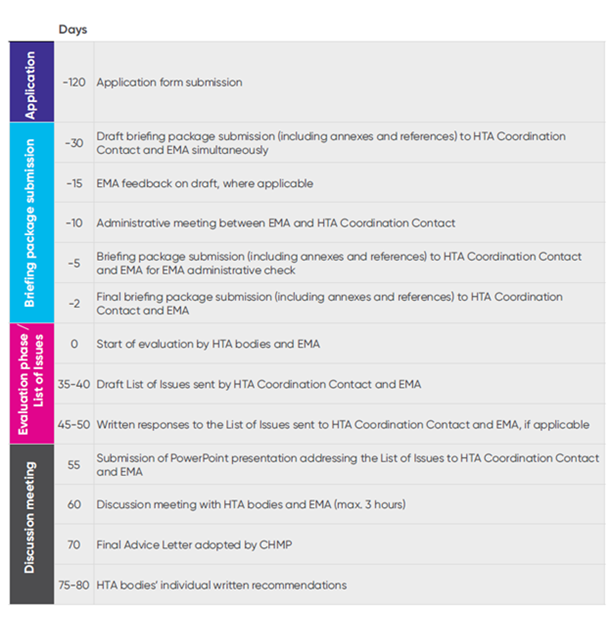
Key: CHMP – Committee for Medicinal Products for Human Use; EMA – European Medicines Agency; HTA – health technology assessment.
Source: European Commission. Guidance on Parallel EMA/HTA body (HTAb) Scientific Advice for the Interim Period. 3 July 2023. Accessed 19 January 2024. https://www.g-ba.de/downloads/17-98-5512/Guidance%20on%20Parallel%20EMA-HTA%20body%20%28HTAb%29%20Scientific%20Advice%20for%20the%20Interim%20Period.pdf
What are the latest developments at the HTA authority level?
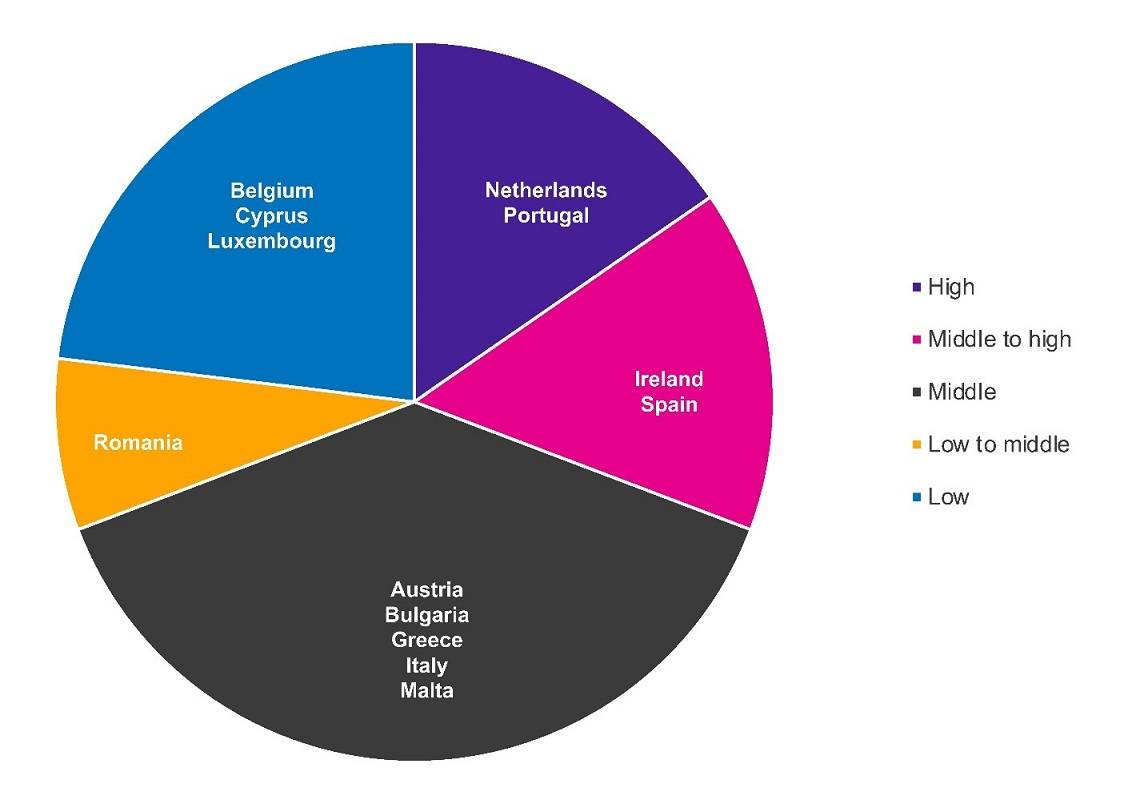
During the interim period, the EC continues to organize regional information events for HTA bodies to discuss the implementation of the JCA and associated challenges on a national level, with an additional 3 meetings planned for 2024. The latest information event was held in January 2023 and included representatives from Austria, Belgium, Ireland, Luxembourg, and the Netherlands. The one prior to that in November 2023 covered Italy, Malta, Portugal, and Spain. These countries rated their readiness to implement the HTAR on a broad spectrum from low to medium and high. High ratings (e.g. Netherlands, Portugal) were mainly based on the strength of the countries’ national HTA systems and past involvement in European initiatives (see Figure 2 for the self-rating of preparedness for the EU HTA by national HTA authorities stated during the information events). However, all the countries still expressed uncertainty on how the JCA will impact national procedures and timelines. The implementing act on the JCA of medicinal products, which was planned to be published by the EC in Q4 2023 and was now postponed to Q1 2024, is an important milestone for countries and will provide final guidance and implications for national implementation. Similar to previous information events with different EU HTA bodies, the main challenges highlighted by representatives from the 9 countries were resource constraints and capacity building to adequately conduct EU HTA-related tasks. The overall perception was that countries are eager to adopt the European JCA report with the aim to gain efficiency. However, countries will likely adopt a flexible approach to learn how to use the JCA reports and adapt local processes over time.
Figure 2. Self-rating of preparedness for the EU HTA by national HTA authorities
Sources: 1. European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2024. Accessed 30 January 2024. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2024-01-30_en. 2. European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2023. Accessed 22 November 2023. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2023-11-22_en. 3. European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2023. Accessed 18 September 2023. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2023-09-18_en.
Conclusions
To conclude, biopharma companies, medical device companies, and HTA bodies are currently awaiting the JCA and JSC implementing acts for final guidance to take appropriate actions for efficient preparation for the processes. While oncology drugs and ATMPs will be the first medical products to be affected by the JCA, manufacturers of non-oncology/ATMP drugs and medical device companies should also consider starting to initiate strategic JCA-related activities, as well as participate in JSCs to inform their pivotal trials in early planning phases. For local HTA bodies, it will be crucial to advance capacity building to have sufficient resources available by the end of 2024 before the first JCA procedures start. Close collaboration between all stakeholders will be crucial already during the interim period to ensure efficient JCA and JSC processes, even though the implementation of the EU HTA will likely be a developing system with adaptations made based on learnings from the first processes conducted.
Upcoming events:
- 8 March 2024: Meeting of the HTA Coordination Group
- 9 April 2024: Info day for HTA bodies – Estonia, Latvia, Lithuania, and Poland
Sources
EUnetHTA. Joint HTA Work. 2023. Accessed 17 January 2024. https://www.eunethta.eu/jointhtawork/
EUnetHTA. EUnetHTA 21 – Stakeholder meeting. 8 September 2023. Accessed 27 September 2023. https://www.eunethta.eu/wp-content/uploads/2023/09/EUnetHTA-21-Stakeholder-Meeting-08.09.2023-v0.2.pdf
EUnetHTA. Frequently asked questions (FAQs) regarding EUnetHTA 21 Joint Scientific Consultations (JSC). 2021. Accessed 17 January 2024. https://www.eunethta.eu/jscfaq/
European Commission. Guidance on Parallel EMA/HTA body (HTAb) Scientific Advice for the Interim Period. 3 July 2023. Accessed 19 January 2024. https://www.g-ba.de/downloads/17-98-5512/Guidance%20on%20Parallel%20EMA-HTA%20body%20%28HTAb%29%20Scientific%20Advice%20for%20the%20Interim%20Period.pdf
European Commission. The EU regulation on health technology assessment: what’s in it and why it matters? 22 November 2023. Accessed 19 January 2024. https://health.ec.europa.eu/system/files/2023-11/hta_20231122_co01_en.pdf
European Commission. Summary report – HTA Stakeholder Network (17 November 2023). 11 January 2024. Accessed 19 January 2024. https://health.ec.europa.eu/latest-updates/summary-report-hta-stakeholder-network-17-november-2023-2024-01-11_en
European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2023. Accessed 18 September 2023. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2023-09-18_en
European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2023. Accessed 22 November 2023. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2023-11-22_en
European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2024. Accessed 30 January 2024. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2024-01-30_en
European Commission. From theory to practice: implementing the EU Health Technology Assessment Regulation. 2024. Accessed 14 February 2024. https://health.ec.europa.eu/events/theory-practice-implementing-eu-health-technology-assessment-regulation-2024-04-09_en
G-BA. Europäische Beratungen: G-BA zentrale Kontaktstelle für parallele Beratungen durch EMA und HTA-Agenturen in Europa im Übergangszeitraum bis zur Implementierung der EU-HTA-Verordnung. 2023. Accessed 17 January 2024. https://www.g-ba.de/themen/arzneimittel/arzneimittel-richtlinie-anlagen/nutzenbewertung-35a/informationen-fuer-unternehmen/eu-hta-verordnung/
G-BA. Ändert die Implementierung der EU-HTA-Verordnung das AMNOG-Verfahren? Accessed 20 January 2024. https://www.g-ba.de/service/veranstaltungen/amnog-eu-hta-verordnung/
Additional insights
Insight
HTA Quarterly Fall 2024
AmerisourceBergen
August 2024
In this special themed edition, our colleagues at Vintura, a Cencora company, explore the potential of value-based partnerships (VBPs). First outlining the principles, goals and potential benefits of VBPs, this edition also provides two case studies that demonstrate how successful partnerships work in practice.