Check out what's new in this month's newsletter
- Stay up-to-date on all the recent updates with the AMCP dossier Format v5.0 resources
- Available now: AMCP webinar hosted by FormularyDecisions, “Bracing for the impact of biosimilars: Navigating terrain in the U.S. market landscape”
- New and enhanced features: eRequest update
AMCP dossier Format v5.0 resources
Join our dossier experts for an informal snack-and-learn
The Cencora team has received many questions regarding the changes to the AMCP Format and how it will affect current and future dossiers. Our AMCP dossier experts are offering an informal 30-minute call to provide an overview of the key changes within AMCP v5.0 and to answer any questions that you may have regarding this update.
We are also able to share insights into opportunities for new evidence generation and product differentiation with the new Format.
Interested in learning more? Connect with one of our dossier experts.
AMCP webinar
Session: New and Improved - AMCP Format for Formulary Submissions v5.0
Date: June 25, 2024
Time: 2-3 PM ET
Recording available soon
The AMCP Format for Formulary Submissions (aka, the Format) defines best practices for pharmaceutical manufacturer dossiers. In April 2024, AMCP released the Format v5.0, the first update since 2020. In this educational session, two individuals who contributed to the revision will provide details of the new and improved Format v5.0.
Learning objectives:
- Summarize the AMCP Format and its history
- Identify updates made in the AMCP Format v5.0
- Discuss best practices for manufacturers in AMCP Format dossier development
To access the recording once available visit amcplearn.org
Research presentation:
Payer pre-approval information needs and how manufacturers can better meet them
Here, Ben Penley, Manager, Evidence Generation & Value Communications, answers questions about his research, which was presented at AMCP 2024, on HCDM perceptions of PIE after the passage of the PIE Act.
More information
Interested in learning more? Connect with one of our dossier experts.
Webinars

Did you miss it?
AMCP webinar: Bracing for the impact of biosimilars
Webinar: Bracing for the impact of biosimilars: Navigating terrain in the US market landscape
Recorded on: June 11, 2024
Hosted by: FormularyDecisions
Watch this engaging discussion to discover the keys to thriving in the dynamic biosimilars landscape. In this session, you will gain practical insights on navigating regulatory challenges, seizing opportunities for collaboration, and communicating effectively with stakeholders. Stay ahead of the curve with up-to-date industry trends and expert strategies tailored to driving success in the US biosimilars market.
Learning objectives:
- Review the US biosimilars market landscape and the current legislative and regulatory environment
- Identify key factors affecting payer uptake and provider adoption of biosimilars in the US
- Explore challenges and opportunities associated with biosimilars for stakeholders across the healthcare continuum
- Position yourself for success by leveraging expert strategies and insights to proactively address potential market challenges
New and enhanced features in FormularyDecisions
What's new?
Improvements made to eRequest
Several enhancements were made to eRequest earlier this month, enabling HCDMs to provide clearer, more specific requests to your team. With this update, all eRequests moving forward will include the following detail:
Type of eRequest:
- eDossier requests will specify the disease state of interest, making eRequests easier to fulfill and ensuring the correct information is provided
- Other information requests will be tracked separately from eDossier requests and include a short explanation from the HCDM
Follow-up preference:
- HCDMs will also indicate whether they would like a follow-up meeting to discuss their request
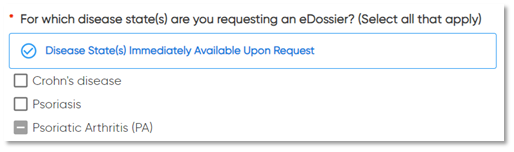
This eRequest update has already been reflected in your eRequest emails and inbox notifications. Additional improvements will also be released into your Insights Dashboard later this summer, including enhanced visuals that reflect the new level of detail behind each eRequest.
Don't hesitate to contact us below if you have any questions about the eRequest updates.



