The Value of Real-World Evidence in Canadian Drug Commercialization: Part 2
By AmerisourceBergen
Now more than ever, Canadian payers and regulators find
value in outcomes-based evidence beyond randomized controlled trials in
regulatory and reimbursement decision-making. Just last year, Health Canada
communicated how they plan to optimize the use of high-quality real-world
evidence (RWE) for regulatory decisions; the Canadian Agency for Drugs and
Technologies in Health (CADTH) recently shared their RWE future strategy,
including advice, guidance, evaluation, and collaboration parameters; and
payers continue to look to RWE for reimbursement decisions, all providing
opportunities for RWE that didn't exist just years ago.
As these trends have taken shape, manufacturers have
increased efforts to generate data from real-world settings through patient
support programs (PSPs); however, there is an increasing need for manufacturers
to collect real-world data from multiple sources across the care continuum to
support reimbursement across the drug lifecycle.
New and existing sources for real-world evidence
- Patient-reported data from validated questionnaires
- Clinical data from provider-site electronic medical records or patient charts
- Claims data from payers (public or private)
- Manufacturer-sponsored patient support program data
- Pharmacy dispensing data and wholesale data
- Patient registries
- Lab testing
- Digital data sources, including mobile healthcare apps and wearables
In fact, as is evident in Figure 1 below, which illustrates various data collection points across a patient journey for a specialty product, there are multiple opportunities to collect a variety of real-world data along the continuum.
Figure 1
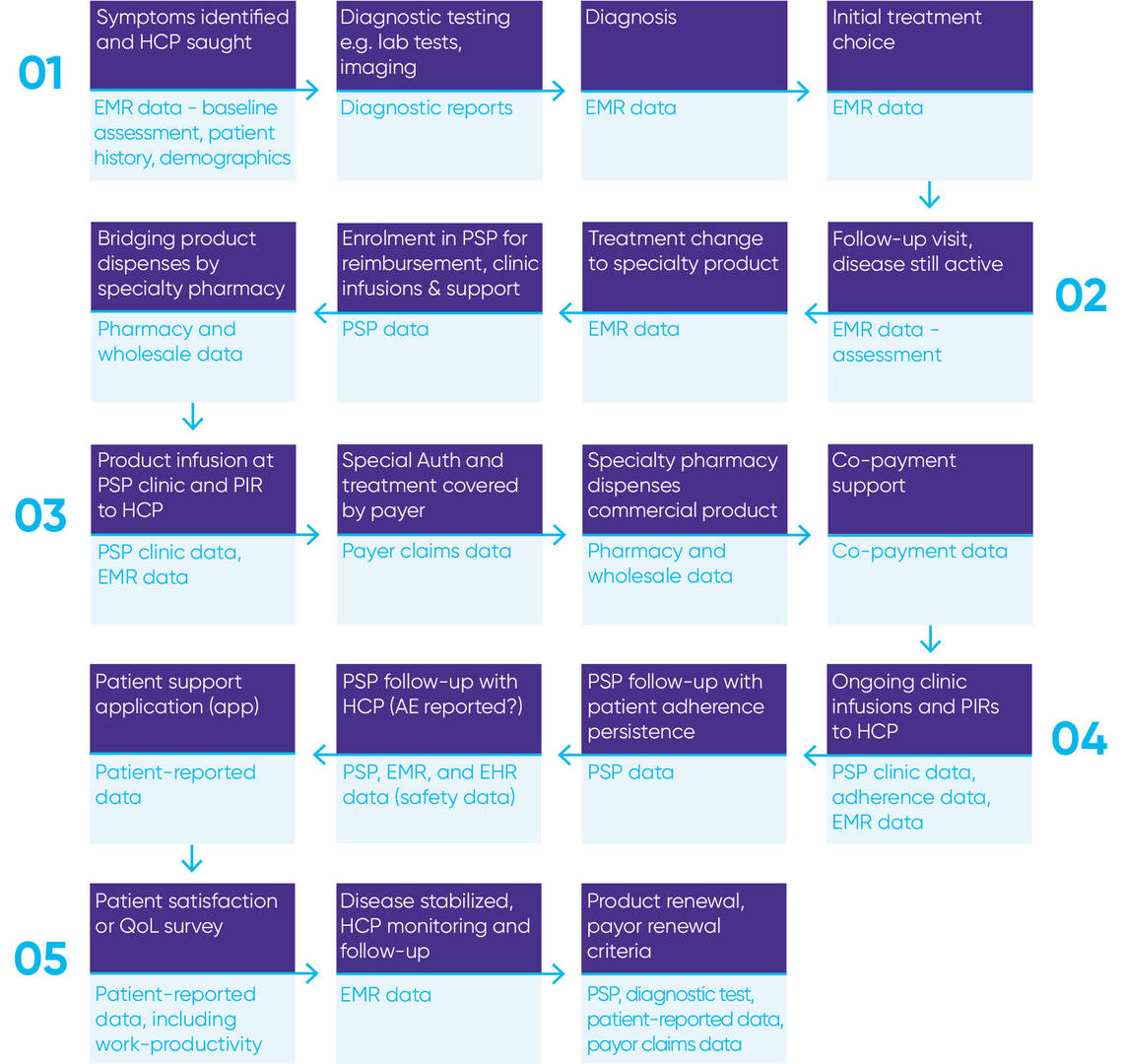
Having such an expansive and diverse net of data collection generates more meaningful insights.
The need for data linkage
Despite the promise of data diversity and robustness, joining disparate sources into a unified aggregated data set can be challenging.
Healthcare system fragmentation produces an incomplete picture of the patient, but linking the various sources together provides a more accurate characterization.
Unfortunately, the current health data landscape can make it difficult to bring data sets together.
Solving these challenges requires a data enrichment strategy that's able to aggregate the different types of RWE across the care ecosystem ¾for example, the analysis of prescriptions along with reporting of adverse events, or the analysis of adherence and its impact on long-term patient health outcomes. Data linkage and aggregation can help address the shortcomings of single-source data capture while also bolstering the scientific quality and validity of research studies. Linking together these sources helps manufacturers and providers alike to assemble a more holistic view of the patient and expand the set of questions that can be answered on the effectiveness and overall value of treatments.
Another opportunity in data linkage comes from public sources, as the Canadian healthcare model itself creates new opportunities for collaborative partnerships around analysis. Through this linkage of governmental data with information on health service utilization (such as hospital stay, ER use and outpatient and physician visits), manufacturers can glean more comprehensive insights that are invaluable to understand the patient journey and outcomes.
Manufacturer opportunities
While payers and regulators seek to facilitate the adoption of RWE, manufacturers are taking a leadership position in finding, generating, and contextualizing that evidence.
Simply put, the government and the payers are very open to RWE, but they want manufacturers to drive RWE solutions (particularly for adoption and continued use of the manufacturer's products).
As Figure 1 demonstrates, PSPs are an integral part of the patient journey. They enrich existing information because of the large enrollment/patient capture and multiple touchpoints with patients, which are all opportunities to collect data. PSPs also have direct communication with patients' healthcare team and physicians, payers, and caregivers ¾ representing even more opportunities for data collection.
Data that resides within the PSPs remain critical for fully understanding RWE. One way to ensure the PSPs are structured to support RWE collection is by creating a strategy that prioritizes patient-reported data. One aspect of patient support programs that can be particularly useful is contacting the patients directly to gather information. Additionally, as digital health solutions such as app and wearables increase in utilization, personal technologies represent another source of continuous data collection that should be considered. When setting up PSPs, it is also important to ensure the quality and accuracy of the data by investing in validated quality control systems to provide confidence in the credibility of the data collected.
Successful commercialization strategies will utilize and incorporate real-world evidence across the product life cycle, and the need to link data to forecast and track outcomes over time. Especially in the new era of COVID-19, RWE will be critical in measuring and understanding the long-term impact of the disease, which in turn will help create a treatment roadmap and allow us to expedite launching therapies across the world.
Working with an experienced vendor in the nuances of data analysis and linkage can enable manufacturers to achieve their commercial initiatives and improve patient and health outcomes.