InnomarLive 2019: Commercializing in Canada
By AmerisourceBergen
A recap of the 10th annual InnomarLive conference, covering key topics impacting commercialization in Canada.
On November 28 2019, InnomarConsulting™ hosted its 10th InnomarLive Conference Services, entitled “Commercializing in Canada: Synergies between regulatory and health technology agencies”. Chaired by Durhane Wong-Reiger, President and CEO of the Canadian Organization for Rare Disorders (CORD), the full day event included presentations and panel discussions from key Canadian opinion leaders on topics ranging from regulatory, health technology agencies (HTAs), private payers, industry, and patient advocacy. Over the course of the day, participants heard about the transparent and collaborative efforts that have been launched to accelerate access to therapeutic products, the importance of timely access to innovative medicines for patients through tools such as real world evidence, and the key steps to a successful commercialization journey.
Health Canada and the HTA agencies have demonstrated significant efforts to accelerate the complex process of getting new specialty medicines to Canadians.
Megan Bettle, Director, Centre for Regulatory Excellence, Statistics and Trials at the Biologics and Genetic Therapies Directorate of Health Canada, outlined the plan for “an agile regulatory system that supports better access to therapeutic products based on healthcare system needs”. The plan includes: expanded collaboration with health partners including the Canadian Agency for Drugs and Technologies in Health (CADTH), through the alignment of the HTA review, called “R2D2”, and implementation of a mechanism for early parallel scientific advice; more timely access to drugs and devices; and enhanced use of real-world evidence.
Trevor Richter, Director of Pharmaceutical Reviews at the Canadian Agency for Drugs and Technologies, CADTH, spoke further about the aligned review process and its two elements: filing pre-notice of compliance (NOC), and manufacturer consent for information sharing between Health Canada and the HTAs. Although this is not a mandatory process, filing early will result in a significant reduction between Health Canada's market approval and HTA recommendations which will lead to more rapid access for patients.
"Filing early will result in a significant reduction between Health Canada's market approval and HTA recommendations."
Sylvie Bouchard, Director, Institut national d’excellence en santé et en services sociaux (INESSS), and Sang Mi Lee, Senior Manager, pan-Canadian Pharmaceutical Alliance (pCPA), each spoke about the focus of their organizations, and efforts to enable commercialization. Sylvie highlighted several targets of Québec’s Life Sciences Strategy including synchronizing the listing recommendations of INESSS and CADTH to an average maximum interval of one month; and the reduction in the time between the NOC and reimbursement by the Québec government. Additionally, the new INESSS assessment framework was described, including a criteria-based evaluative approach, input of patient experiential data, and the concept of potential benefit in certain exceptional situations.
Regulatory considerations that should be top of mind when commercializing a product in Canada were highlighted by Anne Tomalin, VP Quality, Regulatory and Safety, Innomar Strategies. Anne informed the audience on several issues, including Certificates of Supplementary Protection, the new fee structure for Health Canada drug submissions, access to generic drugs, use of real-world evidence, transparency of submissions and appeals, mandatory ADR reporting for hospitals, and updates to Health Canada’s Special Access Programme (SAP).
Canadian regulators, HTA bodies and payers see the value of RWE in gauging the overall quality and appropriateness of health care.
The importance and growing interest in real-world evidence (RWE) was a recurring topic throughout the day. Don Husereau, Senior Scientist with the Institute of Health Economics, spoke about utilization of RWE in commercialization strategies. Don also spoke about how Canadian regulators, HTA bodies and payers view and value RWE, citing a recent publication, “…health system leaders and payers saw its real value in gauging the overall quality and appropriateness of health care, which may or may not involve medicines.”1
Early engagement with private payers is important for manufacturer submission guidance, and to enable parallel work for private payers.
A panel discussion with key private payer experts, Bobby Currie, Director, Drug Strategy, Great-West Life Assurance; Daria O’Reilly, Lead Health Economist, Pharmacy Consulting, Telus; and Ned Pojskic, Leader, Pharmacy & Health Provider Relations, Green Shield Canada, provided insights on commercialization in the changing environment, and how to best engage with private payers along the market access pathway. Early engagement with private payers was stressed by all panel members for manufacturers to gain submission guidance; and to enable private payers to do as much parallel work as possible, and to understand manufacturer pipelines sooner. Also highlighted was the need to adapt models and collect the relevant information that private payers want to see, productivity and disability in particular.
The opportunities and challenges for Canada’s innovative pharmaceutical industry were described by Pamela Fralick, President, Innovative Medicines Canada (IMC). Pamela spoke about advocating for solutions to mitigate the impact of PMPRB reform that has already resulted in companies making decisions to delay investments, launches and hiring; and to stop clinical trials. She also outlined the opportunities in National Pharmacare, access to drugs for rare disease, and an industry working group to develop policy options.
Download this summary as a PDF.
Also from an industry perspective, industry executive, Eric Tse, and Frédéric Lavoie, VP Access and Government Relations, Pfizer Canada, each spoke about the uncertainty in the changing environment, what this might mean for innovation and the ability to get medicines in the hands of patients, and considerations for charting a way forward to commercializing in Canada.
An important element around commercialization is the important activity of planning the product’s distribution and logistics strategy. Dan Chiasson, President and CEO of the Canadian Association for Pharmacy Distribution Management (CAPDM) provided a robust landscape of the Canadian distribution environment as well as recommendations and considerations for launch success such as flow of products, funds, and data.
Siofradh McMahon, Senior Manager Clinical Translation and Regulatory Affairs, Centre for Commercialization of Regenerative Medicine (CCRM), provided the audience with an understanding of the differences and challenges in commercializing living therapies. Known as Advance Therapeutic Products in Canada, a new pathway was approved in 2019 to facilitate development of a regulatory strategy for these cell and gene therapies. Siofradh advised that the future of living therapies in Canada is promising, and currently, there are approximately 95 cell and gene therapy clinical trials ongoing in Canada.
The patient voice is an important factor in the commercialization journey.
Both Ian Steadman, a patient advocate and CORD board member, and Durhane Wong-Reiger spoke eloquently about the impact and importance of the patient voice in the commercialization journey and the need for timely access to new therapies. Ian spoke about his personal journey as a patient with the rare genetic disorder, Muckle-Wells Syndrome, the extreme challenges he faced, and the significant impact of receiving treatment on his life. With the availability of an effective treatment, his daughter, who also has Muckle-Wells Syndrome, will not know the struggle and challenges that her father has experienced.
Looking at the commercialization journey holistically, Sandra Anderson from Innomar Strategies summarized the perspectives, proactive planning requirements, and considerations for manufacturers for their product launches. She also highlighted the key phases and planning required (Figure 1) during each stage while focusing on the keys to successful commercialization in Canada.
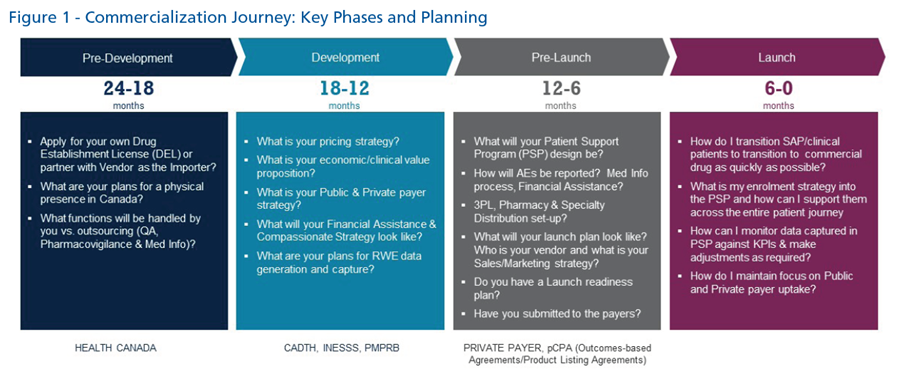
The role of commercialization is a critical step to a successful therapeutic launch. However, there are many elements and activities that need to be considered such as planning early, and engaging stakeholders, clinicians and patients, which can help to optimize timelines, avoid delays to revenue generation, and accelerate patient access to medication.


