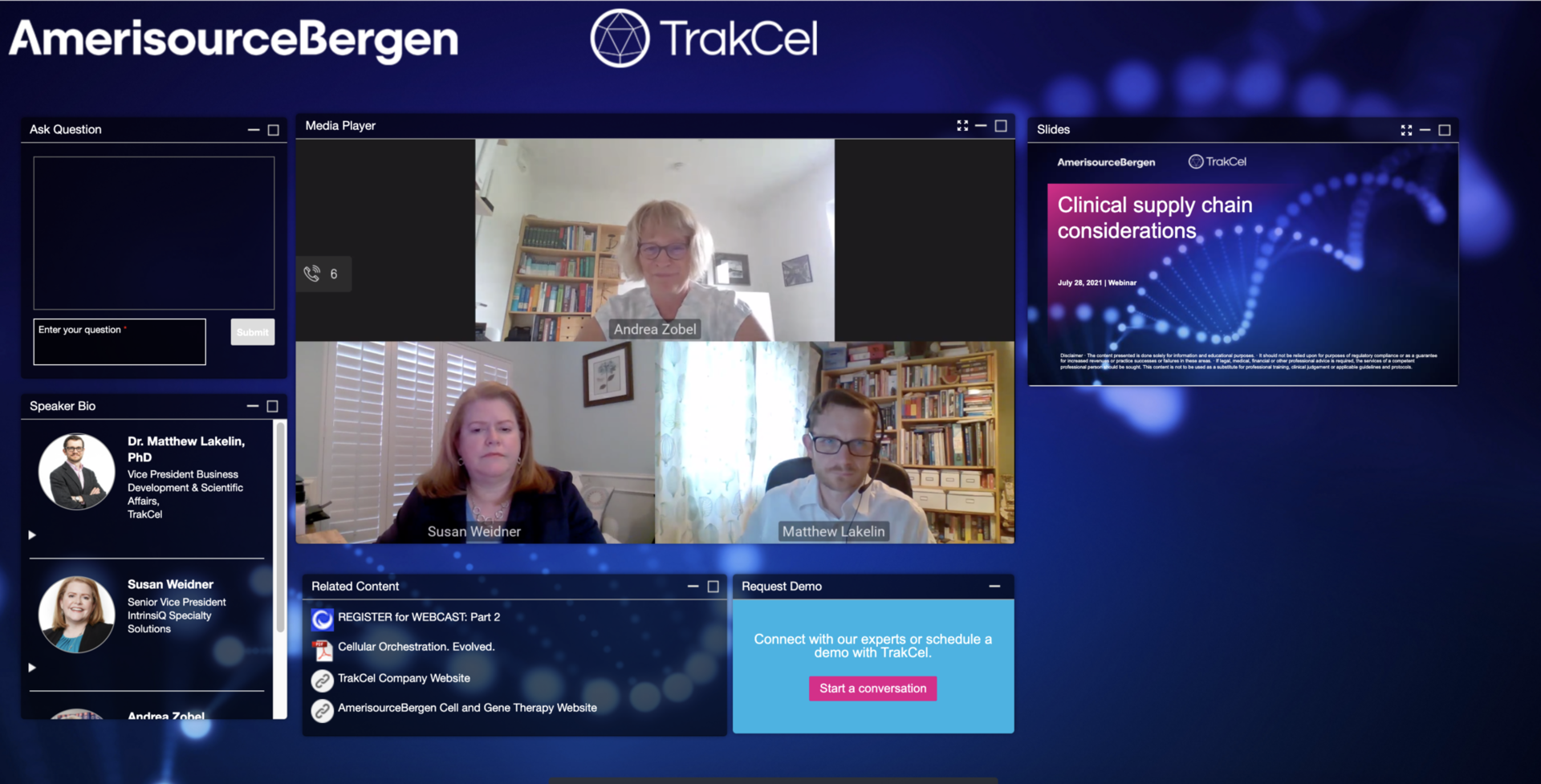
Webinar | Cell and gene therapies: Clinical supply chain considerations
By AmerisourceBergen
Key elements to
consider during clinical development for an optimal cell and gene therapy
supply chain and tracking outcomes
Novel cell and gene therapies require a different approach to the clinical supply chain. AmerisourceBergen and TrakCel experts will discuss key considerations for product owners during clinical development. Hear lessons learned from the latest cell and gene therapy clinical trials and insights on what you need to know about the clinical development journey.
In this webinar, you’ll learn:
Click below to watch the webinar on demand.
In this webinar, you’ll learn:
- What to consider when evaluating global transport solutions
- The best approach to partnering on clinical trial design
- How to make the most of technology and tracking in the clinical phase
Click below to watch the webinar on demand.
Moving healthcare forward
When healthcare advances, so must the processes to connect patients to the therapies they need. And because a rapidly changing cell and gene therapy industry requires an equally innovative partner, AmerisourceBergen is committed to delivering solutions that enable this connection.
Let’s build the future together.