Q. How will my pharmacy and patients be impacted?
A. Though many of the procedures you currently practice already satisfy the requirements of the Injunctive Relief agreement, we will be making some adjustments to our Controlled Substance Monitoring Program (CSMP). These changes create consistency in approach across the three largest wholesale distributors.
Q. What will be changing?
A. There are a few key changes to our Controlled Substance Monitoring Program (CSMP):
- We expect a higher quantity of order lines to be flagged by our suspicious order monitoring system. All flagged order lines will be automatically cancelled and reported—the rest of your order (if applicable) will still ship. We are adjusting the way our algorithm flags suspicious orders. The new algorithm will flag order lines of unusual size, frequency or pattern based on a pharmacy’s own order history or when compared to peers. Previously, AB would review flagged orders and either cancel or approve for shipping. We will no longer perform that review process. Any order lines that are flagged by the algorithm will be automatically cancelled and reported.
- As part of our due diligence, we will be making unannounced pharmacy visits to observe and speak with the responsible pharmacist. Previously, we have notified you in advance of on-site pharmacy visits. As part of the settlement agreement, we are required to make unannounced visits to observe the pharmacy activity and we may need to ask questions of the lead pharmacist. Those visits may be conducted by our third-party partner, Pharma Compliance Group (PCG). PCG representatives will be given credentials to show that they are affiliated with AB and you may also contact your Sales Representative if you would like additional confirmation.
- We will be required to collect 90 days of de-identified dispensing data from you more frequently. Please ensure you have processes in place to efficiently turn over this data to AB or our HIPAA-compliant technology vendor, Pro Compliance, when requested. See the AB Dispensing Data Requirements document to understand what data/information will need to be provided.
Q. Will my entire order be cancelled including non-controls if it’s flagged?
A. No. If you submit an order that contains both controlled substances and other products (Rx, OTC, etc.) and lines within your order are flagged and cancelled, the portion of your order that does not contain controlled substances will still ship and not be impacted. If you have an order for controls with multiple lines across different drug families, only those order lines that exceed established thresholds for a particular drug family would be flagged and cancelled. The remaining controls that did not exceed the threshold will still ship.
Q. Where can I see if my order lines were cancelled because they were flagged
A. This will show on the item line on your invoice or in ABC Order within the order details as “Rejected due to OMP.” See the image below for an example:
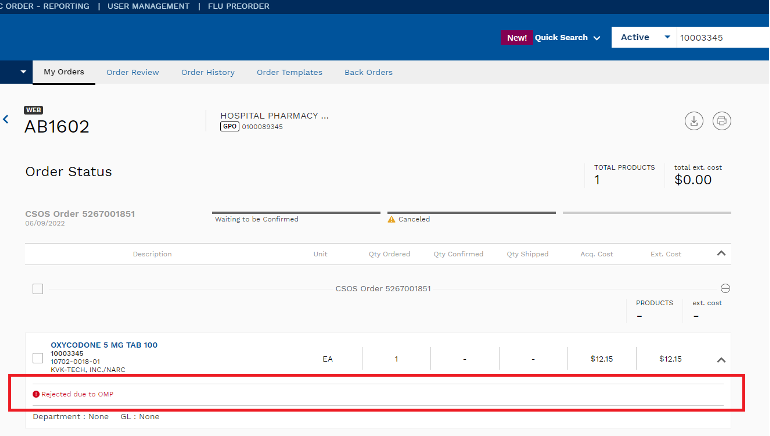
Q. When will the changes go into effect?
A. July 1, 2022.
Q. What customers are subject to the new Controlled Substance Monitoring Program changes?
A. All customers who are registered with the DEA as a Retail Pharmacy, including independents, chains and mail order pharmacies.
Q. What facilities are out of scope for the new Controlled Substance Monitoring Program changes?
A. Closed door retail pharmacies servicing long-term care and hospice patient communities. Hospital inpatient pharmacies. and Clinics, physician practices, distributors and researchers
Q. If I switch to McKesson or Cardinal, will I still be subject to these new requirements?
A. AmerisourceBergen, McKesson, and Cardinal have all entered into the same agreement and are each subject to the exact same requirements.
Q. What if I am routinely having order lines flagged and I’m unable to meet the needs of my patients?
A. If you are routinely having order lines flagged and cancelled and are unable to meet the legitimate medical needs of your patients, there is a process where you may request a threshold review. You must provide comprehensive justification for your request, and we will conduct appropriate due diligence to determine whether a threshold adjustment is warranted. Threshold reviews will generally not be considered for customers who have a relatively small number of order lines cancelled by our system.
You may request a threshold review by contacting Customer Service who will provide you with the correct form and instructions for submitting the request.
Q. If you report my orders exceeding thresholds to regulators, will I get in trouble?
A. There are several reasons why your orders may exceed established thresholds. If you experience multiple cancelled orders in a short period of time and it is impacting your ability to serve the legitimate medical needs of your patients, you may request a threshold review so the Controlled Substance Monitoring Program (CSMP) team can potentially make adjustments. AmerisourceBergen does not have visibility into any review that regulators may perform regarding reported orders.
Q. Why do I have to report more data to you when you already have a record of my orders?
A. While we have a record of your orders and shipments from us, there will be times when we will need to evaluate patient de-identified dispensing data for controlled substances. This data includes information about total dispensing by NDC, cash payments for controlled substances, top prescribers of certain high-risk controlled substances, and out of area patients served by your pharmacy.
See the AB Dispensing Data Requirements document to understand what data/information will need to be provided.
Q. Will you be making visits to my pharmacy?
A. Pharmacy visits may be necessary as part of this process. We understand you’re busy and will make every effort to be sensitive to and respectful of your time in the event a visit is necessary. Historically, we have notified you in advance if we needed to do an on-site pharmacy visit to conduct due diligence. As part of the settlement agreement, we are required to make unannounced visits to pharmacies to observe the pharmacy activity and we may need to ask questions of the lead pharmacist.
We will have a process in place where you can authenticate the site visit representative is with AB or our third-party partner, Pharma Compliance Group(“PCG”), prior to engaging with them on-site.
Q. How will your compliance with these requirements be tracked?
A. AmerisourceBergen will provide regular reports to our assigned independent monitor.
Q. What if I need to report an issue related to the diversion of controlled substances?
A. Please utilize our EthicsPoint Hotline to report any concerns relating to the diversion of controlled substances. EthicsPoint is a comprehensive and confidential reporting tool managed by a third-party partner that allows us to proactively address potential instances of fraud, abuse, and other misconduct.
Q. Why did you agree to this settlement? Did you do something wrong?
A. AmerisourceBergen has always had robust programs in place to meet all regulatory requirements. The opioid epidemic has resulted in nationwide lawsuits, and as a result, we decided it was in the best interest of our company, customers, and patients to enter into this agreement to expedite the flow of resources into communities impacted by the crisis while allowing us to continue to ensure the pharmaceutical supply chain meets the needs of healthcare providers and patients.
