Check out what's new in this month's newsletter
- Congratulations to recipients of our 2024 Innovation & Impact Awards!
- Now live: AMCP Dossier Format Version 5.0 release
- Register for the upcoming AMCP webinar hosted by FormularyDecisions, “Bracing for the Impact of Biosimilars: Navigating Terrain in the U.S. Market Landscape”
- New and enhanced features and offerings:
- New Automated Implementation package - Cheers to 5 years: FormularyDecisions 5-year anniversary
AMCP Dossier Format Version 5.0 is live

Missed us at AMCP Annual?
One of the topics discussed in our FormularyDecisions session, The Role of Unapproved Product Dossiers in a Successful Product Launch, was AMCP’s recent Dossier Format 5.0 release.
Key updates include:
- Special content considerations for digital therapeutics
- The addition of health disparities related to social and demographic factors
- Increased emphasis on inclusion of real-world evidence
Additionally, the guidance places importance on brevity to streamline dossiers and add-on guidance for pre-approval information exchange. Click below for full details on the AMCP Format 5.0.
Also, if you're interested in learning more, click below to connect with one of our dossier experts.
Upcoming webinars

Session:
Date: June 11, 2024
Time: 2-3 PM ET
Join this interactive, engaging, and informative discussion to discover the keys to thriving in the dynamic biosimilars landscape. In this session, you will gain practical insights on navigating regulatory challenges, seizing opportunities for collaboration, and communicating effectively with stakeholders. Stay ahead of the curve with up-to-date industry trends and expert strategies tailored to driving success in the U.S. biosimilars market.
Learning objectives:
- Review the U.S. biosimilars market landscape and the current legislative and regulatory environment.
- Identify key factors affecting payer uptake and provider adoption of biosimilars in the U.S.
- Explore challenges and opportunities associated with biosimilars for stakeholders across the healthcare continuum.
- Position yourself for success by leveraging expert strategies and insights to proactively address potential market challenges.
Click below for webinar details and to register through the AMCP website:
Congratulations to our FormularyDecisions | Cencora 2024 Innovation & Impact award recipients:
AbbVie (Mid-to-large biopharma category) and SpringWorks Therapeutics (Emerging biopharma category)

It is awarded annually to distinguished recipients in 2 categories: emerging biopharma and mid-to-large biopharma. Evaluation criteria encompass (1) biopharma engagement on the FormularyDecisions platform demonstrated via a range of factors, including materials shared with HCDMs; and (2) HCDMs engagement via interaction with the company’s product on FormularyDecisions.


From left to right: Bonnie Dean, Senior Director, Customer Engagement & Operations, FormularyDecisions; Wes Matthias, AbbVie; Gina McKnightSmith, AbbVie; Charlene Kaiser, SpringWorks Therapeutics; Robert (Neil) Nebughr, SpringWorks Therapeutics; Laurie Fazio, Head, Manufacturer Strategy, FormularyDecisions; Jasmine Knight, Senior Director, FormularyDecisions
From left to right: Bonnie Dean, Senior Director, Customer Engagement & Operations, FormularyDecisions; Wes Matthias, AbbVie; Gina McKnightSmith, AbbVie; Charlene Kaiser, SpringWorks Therapeutics; Robert (Neil) Nebughr, SpringWorks Therapeutics; Laurie Fazio, Head, Manufacturer Strategy, FormularyDecisions; Jasmine Knight, Senior Director, FormularyDecisions
Cheers to 5 years
A legacy of innovation with FormularyDecisions
In May 2019, Xcenda, a subsidiary of Cencora, acquired Dymaxium, the company behind the development and launch of FormularyDecisions. Once an exclusive eDossier hosting platform, FormularyDecisions has evolved to meet the needs of biopharma companies and healthcare decision makers over the last 5 years we have built upon our offerings to subscribers, expanded out reach through our partnership with AMCP, and created access to additional resources for HCDMs that can streamline the search for product information.
Over the past 5 years, there has been a more than 8-fold increase in HCDM activity:
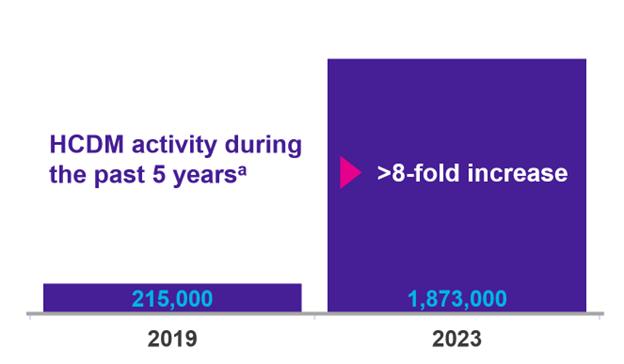
aFormularyDecisions data on file, 2019 and 2023.
Creating access to additional resources
In 5 years, FormularyDecisions has added an industry-recognized Biosimilar Hub, Cell & Gene Therapy Hub, Product Snapshots, Clinical Guideline Snapshots, Formulary Coverage Insights, and MyInsights Program Surveys and Feedback opportunities.
New and enhanced features in FormularyDecisions
Automated Implementation packages now live
What's new?
As of May 23, FormularyDecisions began using the new Automated Implementation Packages with our subscribers for subscription setup and renewals.
The new Automated Implementation Package replaces the legacy Excel spreadsheet implementation package with a more streamlined and user-friendly interface and will improve your experience with completing the package.
Streamlined process and enhanced user experience
- No more manual data entry and complex spreadsheets. Our Automated Implementation Packages leverage the Power Platform to simplify the setup process.
- Save time and increase accuracy with the intuitive interface.
- Designed with you in mind: enjoy a seamless and user-friendly experience from start to finish.
- Clear instructions, visual cues, and smart automation ensure minimal effort for package completion.
- Automated Implementation packages are now conveniently shared as links, and you can revisit them anytime, before or after submission.
Questions around Automated Implementation package?




