What's in store for 2024

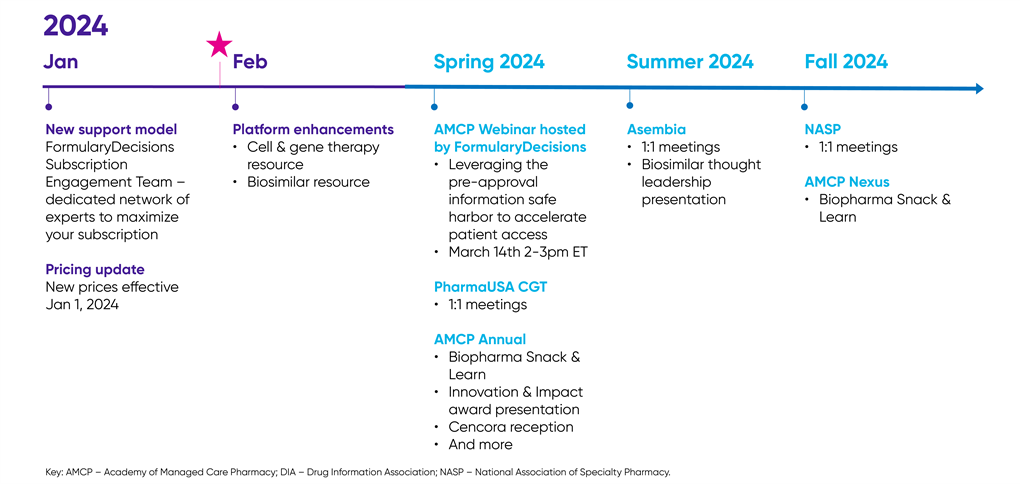
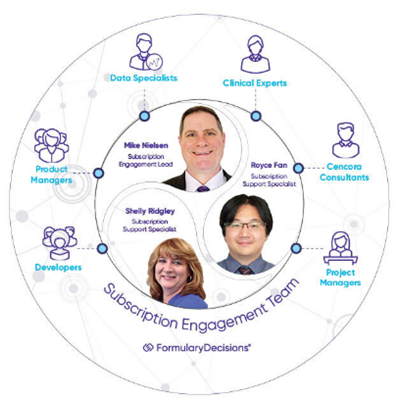
Launch of your Subscription Engagement Team
Your SET will assist with an array of activities—from setting up your subscription, digitizing your eDossier, providing technical support, as well as helping address any other questions that may arise. The new centralized support model is designed to provide a simplified solution to your needs with your user experience as our priority!
FormularyDecisions SET – your personalized support backed by a broad, cross-functional team, and a suite of self-service tools.
Important reminder:
2024 pricing effective January 1, 2024

*Annual pricing per product subscription reflects pricing effective on January 1, 2024.
† Each product subscription includes HCDM insights for 1 disease state. Pricing for each additional disease state is based on the modules included in the subscription (eg, for a product subscription with all 3 modules: $10,000 per additional disease state; for a product subscription with Payer Landscape and eDossier Plus modules: $7,500 per additional disease state).
Note: For all existing subscriptions that are renewed before March 31, 2024, or new subscriptions that entered contracting and signature processes before January 1, 2024, the 2023 pricing will be honored.
New and enhanced features in FormularyDecisions
Cell and Gene Therapy
This new resource provides healthcare decision makers centralized access to information on cell and gene therapies across the product lifecycle to help inform coverage decisions.

Biosimilars
This comprehensive resource has been updated to include a new indicators tool designed to help health care decision makers quickly compare the indicators of biosimilars and their innovator products, gaining deeper understanding and informing confident coverage decisions.

Upcoming webinar(s)
Accelerate patient access with pre-approval information
Session: AMCP Webinar hosted by FormularyDecisions – Leveraging the pre-approval information safe harbor to accelerate patient access
Date: March 14, 2024
Time: 2-3 PM ET
Join Cencora thought leaders for an interactive, engaging, and informative discussion as they dive into the evolving landscape of PIE and explore opportunities and innovative solutions for for leveraging PIE when collaborating with stakeholders.
Learning objectives:
- Understand how PIE has been leveraged by biopharma companies to communicate with healthcare decision makers through various channels
- Explore the impact of effective PIE strategies on stakeholder engagement and decision making
- Discuss potential solutions for challenging areas in PIE such as FDA expedited approval pathways and availability of economic/pricing data
- Understand how digital solutions can be leveraged for PIE and its implications for communication with stakeholders
Click below for webinar details and to register through the AMCP website
Upcoming conference(s)
We're going where the payers are!
Expect to see us at AMCP Annual as well as other major conferences such as Asembia, Drug Information Association (DIA), National Association of Specialty Pharmacy (NASP), AMCP Nexus.
Connect with us at AMCP Annual 2024 in New Orleans April 15-18
More details will follow, but here’s a high-level overview of what we’ve got in store:
- Biopharma Snack & Learn
- Innovation & Impact award presentation
- Cencora reception – location and details TBD (click below to get notified)
- Health care decision maker networking event (closed event for healthcare decision makers only)
Here's a sneak peek of some of the activities we have planned by conference in 2024:
- PharmaUSA (March 26-27, Philadelphia, PA): 1:1 meetings
- Asembia (April 28-May 2, Las Vegas, NV): 1:1 meetings, Biosimilars podium presentation
- DIA (June 16-24, San Diego, CA): 1:1 meetings, Biosimilars podium presentation
- NASP (October 6-9, Nashville, TN): Healthcare decision maker networking event (closed event for healthcare decision makers only)
- AMCP Nexus (October 14-17, Las Vegas, NV): Biopharma Snack & Learn, Health care decision maker networking event (closed event for healthcare decision makers only)
Check back for additional details and to see where else we’ll be in 2024.
2023 Highlights

eDossier Plus module update

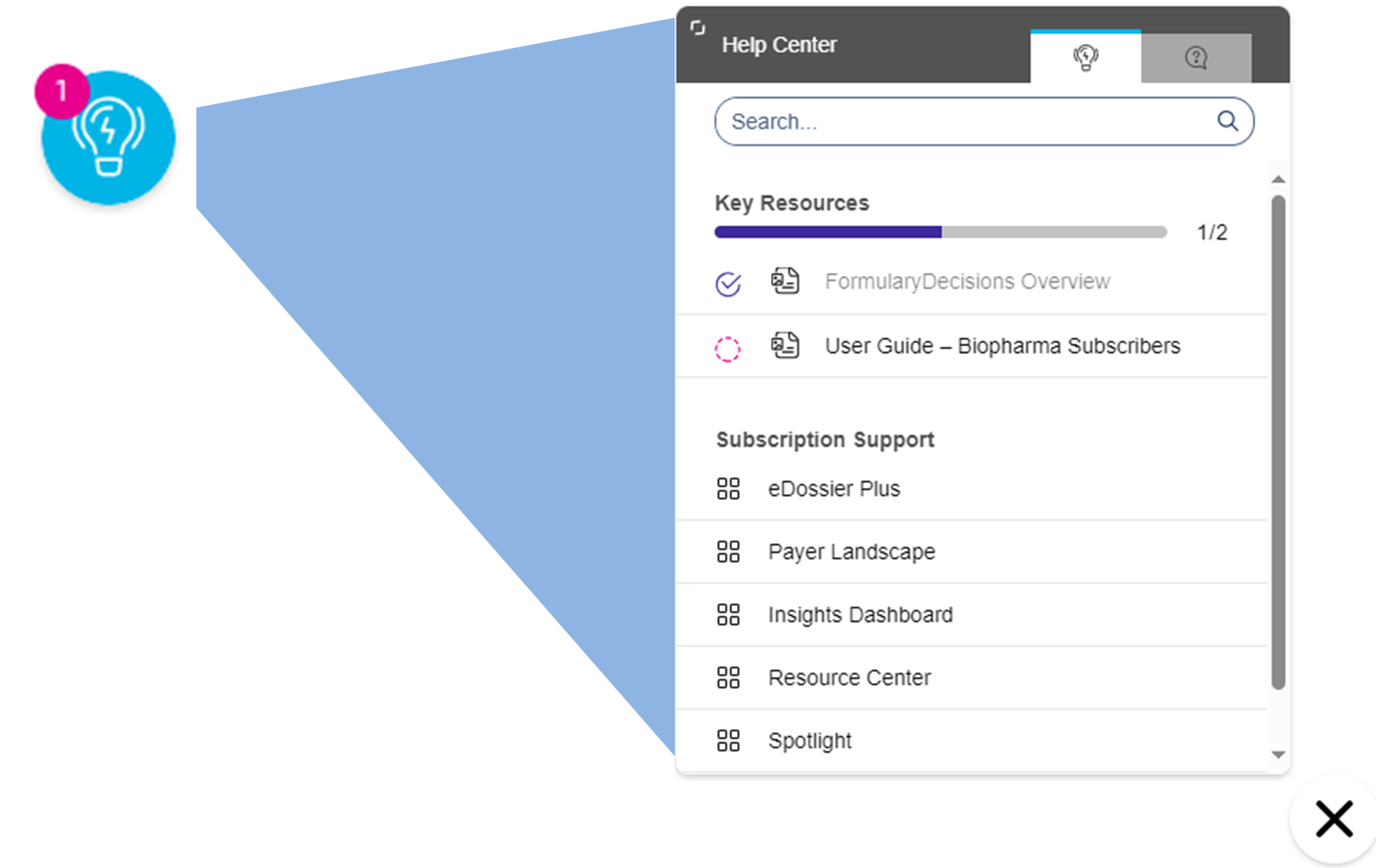
Help Center



