Navigating DSCSA compliance
By AmerisourceBergen
Ensure you're prepared by establishing standard operating procedures
In the pharmaceutical supply chain, dispensers are the final gatekeepers before prescription medications reach patients. The Drug Supply Chain Security Act (DSCSA) places a significant emphasis on the role of dispensers in maintaining the integrity of prescription drugs, safeguarding patients, and ensuring the authenticity of medications.1
The DSCSA replaces all existing or future state-wide drug track or trace systems with a new federal drug tracing program that uses pedigrees and product identifiers for verification of the drugs being accepted by the buyer.1 Compliance with the act will enable all stakeholders to operate and manage the risks of their supply chains more efficiently.2
This article delves into the indispensable role that dispensers play in the pharmaceutical supply chain under the Drug Supply Chain Security Act (DSCSA), focusing on the significance of their contribution, the tools at their disposal and the critical role of Standard Operating Procedures (SOPs) in securing the drug supply chain.
Understanding the Drug Supply Chain Security Act (DSCSA) requirements
The DSCSA places the spotlight on the integrity of the pharmaceutical supply chain, urging all stakeholders, including dispensers, to play their part in ensuring its security.3
Dispensers, as the final point of contact before medications reach patients, are entrusted with the responsibility of verifying the authenticity and safety of each drug transaction through a process called serialization.1,4 While dispensers are not required to change their inbound receiving process, there should be a process and system in place to verify product identifiers: NDC (GTIN), Serial Number, Lot, and Expiration Date, ensuring that product is not counterfeit or diverted.
Under the DSCSA, dispensers have several specific responsibilities, including:
- Verification: Central to the DSCSA and the role of dispensers is the verification process. Verification is the systematic procedure through which dispensers confirm the authenticity and safety of prescription drugs before they are provided to patients.1
- Investigation and reporting: Dispensers will be required to investigate and report any suspect products to the FDA and trading partners within 24 hours.1,5
- Recordkeeping: Dispensers will also be required to store drug transaction information and history for six years. The transaction information includes the name, strength, and package-size of the product, the National Drug Code (NDC) number, and the lot number.
To prepare for the 2023 DSCSA requirements, pharmacies and other dispensers should start implementing serialization processes now. This includes purchasing scanners and other tools to verify product identifiers, as well as developing standard operating procedures (SOPs) for serialization and reporting.
The absence of a robust SOP framework could expose dispensers to significant risks, both in terms of regulatory compliance and operational efficiency.1,2 Inadequate procedures hinder the ability to navigate the complexities of DSCSA, and among the most pressing risks are audits by regulatory bodies, including state regulatory agencies, such as the Board of Pharmacy, Department of Health, etc. and the Food and Drug Administration (FDA).1,3
The FDA has provided guidance to help stakeholders understand and comply with the DSCSA requirements, including guidance on product tracing, verification, and investigation of suspect products. For more detailed information, you can refer to the FDA's official website.5
Effective SOP implementation
SOPs offer a systematic approach to handling, documenting, and reporting prescription drugs. To establish an SOP, dispensers should consider the following steps:
- Needs Assessment: Begin by assessing the unique needs of your dispenser practice. Understand your workflow, identify vulnerabilities, and determine the areas where SOPs are needed most.
- Components of Effective SOPs: Dive into the key components of SOPs tailored to dispensers:
a. Receiving and Verification: Receiving and verification procedures are integral to ensuring the authenticity and safety of prescription drugs. Dispensers must clearly define these procedures, covering the steps involved in receiving pharmaceutical products and verifying their authenticity. Specifically, these procedures should include:
i. Product identifiers: Specify how to receive or exchange transaction information electronically, including specific product identifiers for each package.
ii. Transaction statements: Outline the process for receiving and verifying transaction statements, ensuring that they align with DSCSA requirements.
b. Recordkeeping: Dispensers should establish comprehensive guidelines for accurate and thorough record-keeping, including:
i. Lot numbers: Detail how to record and maintain lot numbers for each product received, ensuring traceability throughout the supply chain.
ii. Transaction information: Specify the method for documenting transaction information, encompassing product details such as name, strength, package size, National Drug Code (NDC) number, and lot number.
c. Quarantine and investigations: Having defined quarantine and investigations protocols are crucial for handling suspected counterfeit or compromised products. Dispensers should detail the following procedures for quarantining and investigating such products:
i. Suspect product identification: Explain how to identify and segregate products suspected of being counterfeit, compromised, or diverted.
ii. Serialized transaction information: Implement systems and processes to produce serialized transaction information if requested by the FDA, a state regulator, or a trading partner for an investigation.
d. Communication: Communication protocols are essential for swift information exchange with supply chain partners in the case of recalls or other issues. Dispensers should outline clear communication procedures, which may include:
i. Recall notifications: Define how to notify patients and relevant stakeholders in the event of a product recall, ensuring compliance with DSCSA requirements.
ii. Supply chain partners: Establish communication channels and responsibilities for liaising with wholesalers or direct suppliers of drug products, especially regarding the storage and exchange of transaction information.
e. Training and education: Dispensers should incorporate these guidelines into their SOPs to ensure their continuing accuracy and effectiveness. Training and education should cover areas such as:
i. Compliance training: Detail the training programs necessary for staff members to understand DSCSA compliance, including verification procedures, record-keeping, and communication protocols.
ii. Regular updates: Specify the frequency of training updates to keep staff informed about evolving regulations and best practices.
iii. Responsibilities: Define the responsibilities of staff members regarding DSCSA compliance, ensuring that they are aware of their roles in maintaining patient safety and drug integrity.
3. Writing the SOPs: Utilize a consistent structure for each component of your SOPs. Clearly explain the steps, responsibilities, and the reasoning behind each process.
4. Customization: SOPs should reflect your practice's specific needs, while remaining aligned with DSCSA requirements.
Resources
For additional guidance and resources on DSCSA compliance and establishing SOPs, refer to the following:
National Association of Boards of Pharmacy (NABP): Guidelines for preparing for DSCSA requirements, including information about serialization timelines and verification processes
National Community Pharmacists Association (NCPA):
DSCSA checklist and SOP guidance document to help dispensers prepare for compliance.
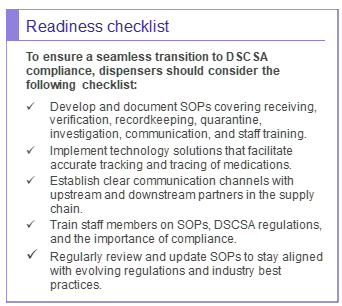
Example SOPs:
https://www.ncpa.co/pdf/sop-track-trace.pdf
https://ncpa.org/sites/default/files/2023-01/ncpa-dscsa-checklist-sop-guidance.pdf https://cdn.ymaws.com/www.mashp.org/resource/resmgr/Resources/DSCSA_v1_Policy_Template.pdf
https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/drug-supply-chain/docs/ASHP-Drug-Supply-Chain-and-Security-Act-Requirements.pdf
https://blog.tracktracerx.com/dscsa-standard-operating-procedure-for-product-tracing
Overcoming challenges and ensuring adherence
Implementing new processes is not without its challenges. Dispensers could face obstacles such as hesitancy to change, operational disruptions, and the need for comprehensive staff training.1 To overcome these hurdles, consider the following steps:
- Leadership buy-in: Garner support from leadership by emphasizing the benefits of SOPs, including improved compliance, patient safety, and operational efficiency.
- Staff training: Invest in comprehensive training programs to ensure that every member of your team understands the importance of SOPs and knows how to execute them effectively.
- Communication: Maintain an open line of communication with your staff throughout the implementation process. Address concerns, answer questions, and provide ongoing guidance.
Conclusion
As the DSCSA goes into effect with a stabilization period, dispensers must recognize that SOP implementation is not just a matter of compliance; it's a commitment to patient safety, drug integrity, and operational excellence.1 By adhering to the principles outlined in this guide, dispensers can navigate the complexities of SOP implementation with confidence.
Embracing SOPs is not just a regulatory requirement; it's a testament to a dispenser's dedication to upholding the highest standards in pharmaceutical care. Remember, in a landscape where patient safety is paramount, robust SOPs for dispensers are not just advisable; they are essential.
References
- "Combating Substandard and Counterfeit Medicines by Securing the Pharmaceutical Supply Chain: The Drug Supply Chain Security Act (DSCSA) of 2013." PubMed, National Center for Biotechnology Information, 16 July 2018, https://pubmed.ncbi.nlm.nih.gov/34007690/.
- "A framework to evaluate interoperable data exchange models for Drug Supply Chain Security Act compliance." Semantic Scholar, Allen Institute for AI, A framework to evaluate interoperable data exchange models for Drug Supply Chain Security Act compliance | Semantic Scholar
- "Drug Supply Chain Security Act (DSCSA)." U.S. Food and Drug Administration, 18 Aug. 2021, https://www.fda.gov/drugs/drug-supply-chain-integrity/drug-supply-chain-security-act-dscsa
- "The Last Mile: DSCSA Solution Through Blockchain Technology: Drug Tracking, Tracing, and Verification at the Last Mile of the Pharmaceutical Supply Chain with BRUINchain." PubMed, National Center for Biotechnology Information, 12 Mar. 2020, https://pubmed.ncbi.nlm.nih.gov/36777051/.
- What do I need to know about supply chain security requirements under the Drug Supply Chain Security Act (DSCSA)? | FDA




