Navigating the way to the new EU JCA—Are biopharma companies, HTA bodies, patients, and clinicians sufficiently prepared?
By Kim Joline Schmidt, MSc, Mareike Konstanski, MSc, Ruairi O'Donnell, PhD, Erika Wissinger, PhD, Herbert Altmann, PhD

The last Stakeholder Meeting under the direction of EUnetHTA 21 in September 2023 has provided some important final insights into the consortium’s deliverables and activities facilitating the implementation of the Health Technology Assessment Regulation (HTAR), including the Interim Joint Scientific Consultation (JSC) process and the Joint Clinical Assessment (JCA) submission dossier template. Procedural and methodological guidelines for the JCA and JSC developed under EUnetHTA 21 still need to be adopted by the Coordination Group on HTA (HTACG). While the implementing act on JCA for medicinal products by the European Commission is planned for the end of 2023, the adoption of procedures related to the JSC will likely not take place until mid-2024.
As the mandate of EUnetHTA 21 concluded in September, the interim period until the full application of the HTAR has started, and, with that, the parallel European Medicines Agency (EMA)/health technology assessment (HTA) body Scientific Advice has been kicked off. Applications for the joint consultations organized by the German Federal Joint Committee (G-BA) have been open since the start of September; however, a period of 6 months is foreseen between application and the advice meeting, meaning that the JSCs during the interim period will start to take place only in 2024. According to the subgroup on JSCs, approximately 3 procedures are planned during the interim period. Participating HTA bodies will be selected during the application process and a minimum of 2 HTA bodies need to be involved for the JSC to take place. During the final EUnetHTA 21 Stakeholder Meeting, it was highlighted that these consultations are focusing on early study advice informing trials that have not yet been initiated at the time of the consultation. In addition to the clinical trial design aspects, the joint consultation may also cover economic questions such as model design; however, final discussions and decisions around costs, budget impact, and cost-effectiveness are not part of the JCA and remain the remit of the national HTA bodies.
In addition to clarifying the JSC procedure during the interim period, EUnetHTA 21 also published the JCA submission dossier template in September. The French Haute Autorité de santé (HAS), the German Institute for Quality and Efficiency in Health Care (IQWiG), and the Irish National Centre for Pharmacoeconomics (NCPE) had a leading role in the creation of the template. The structure of the template and requirements in the JCA submission dossier appear to have many similarities with the current national German HTA submission dossier (see Figure 1 for the topline structure of the dossier). Interestingly, if a health technology has been subject to a JSC by the HTACG, the manufacturer is requested to explain any deviations from the recommended proposition for evidence generation (Section 2.3). This differs from current national procedures in many countries where scientific advice is not binding, and, thus, a rationale for not following early advice does not need to be provided in the national HTA dossier. As for many national HTA submissions, evidence presented in the JCA dossier is expected to be identified and selected systematically, following the international standards of evidence-based medicine (Section 4). Inclusion and exclusion criteria for studies need to be defined upfront and presented for each PICO (population, intervention, comparator, outcomes) separately. Similarly, resulting studies and outcomes are to be shown individually for each PICO (Section 5). Detailed instructions for the compilation of each section are provided in the JCA dossier template.
Figure 1. Topline structure of the JCA submission dossier
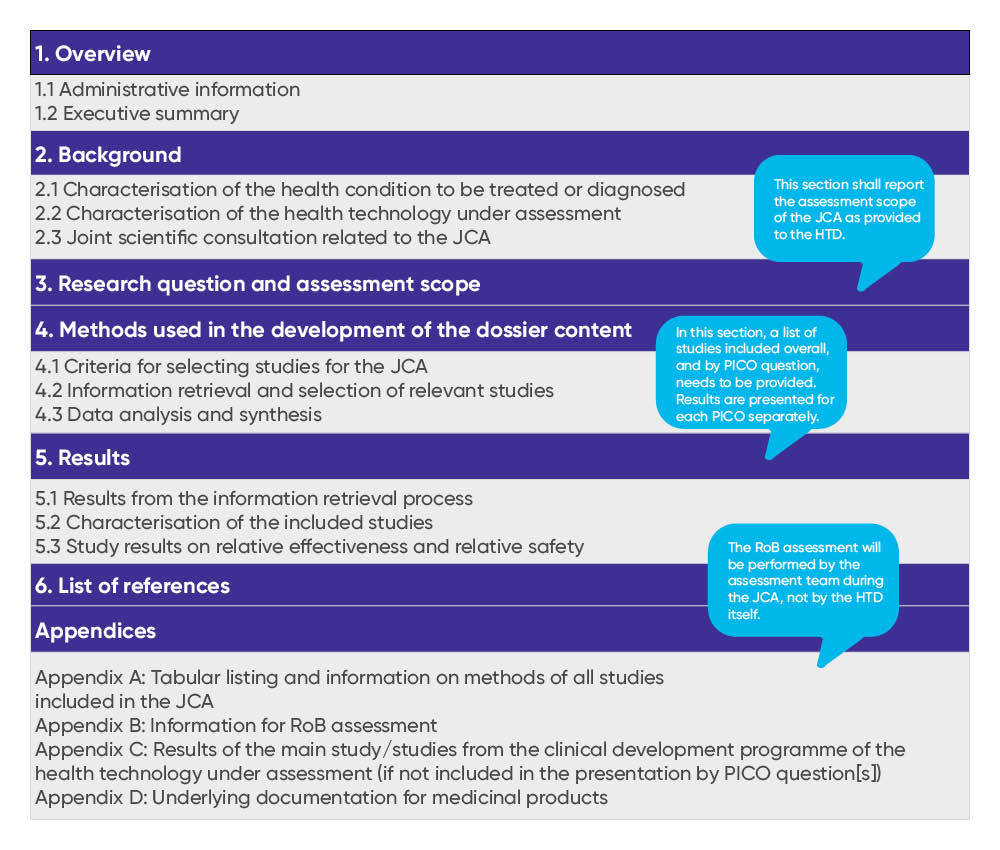
Key: HTD – health technology developer; JCA – Joint Clinical Assessment; PICO – population, intervention, comparator, outcomes; RoB – risk of bias.
Source: EUnetHTA. D5.1 Submission Dossier Template – Medicinal Products. Version 1.0, 31 July 2023. Accessed September 2023. https://www.eunethta.eu/d5-1/
The required comprehensive presentation of available clinical evidence in the JCA dossier may be in line with requirements from most countries; however, local submissions will still need to be adapted to fit each country’s specific needs. It is important to consider that evidence included in the JCA dossier cannot be presented again in national dossiers. To avoid redundancies, national HTA bodies can only request additional analyses that have not been covered in the JCA dossier.
Preparedness of biopharma companies
With clarification of the JSC procedure during the interim period and the finalization of the JCA submission dossier template, more information has been provided to confirm the requirements for biopharma companies. However, there is still a lot of clarity missing and it remains to be seen whether the HTACG will fully acknowledge and adopt the deliverables produced under EUnetHTA 21, while many stakeholders already suggest that important refinements will be necessary.
It will be crucial for biopharma companies to be familiar with the JCA and JSC templates, evidence requirements, and timelines during the interim phase. Internal stakeholder preparation (eg, cross-functional team engagements, upscaling of resources to prepare for parallel EMA/JCA submissions and national processes, statistical analysis) and external stakeholder engagements (eg, participation in joint scientific advice and information events) should be initiated and pursued continuously to optimally prepare for the start of the European Union (EU) JCA in January 2025. Figure 2 provides an overview of key milestones in the preparation of the JCA dossier for biopharma companies.
Figure 2. Key milestones in the preparation of the JCA dossier
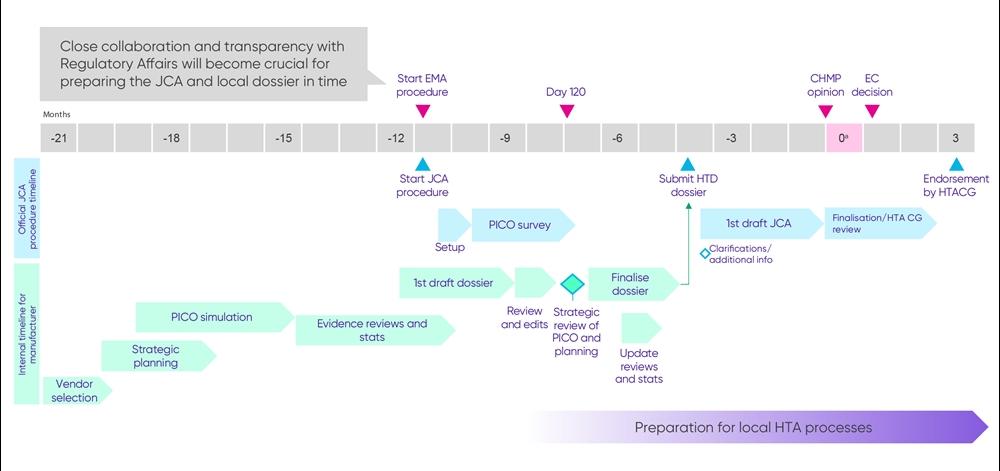
Key: CHMP – Committee for Medicinal Products for Human Use; EC – European Commission; EMA – European Medicines Agency; HTA – health technology assessment; HTACG – Coordination Group on Health Technology Assessment; HTD – health technology developer; JCA – Joint Clinical Assessment; PICO – population, intervention, comparator, outcomes.
a Time zero (T0), months = date of CHMP opinion.
Based on a new medical entity, assuming average regulatory timelines. Representative of published timelines by EUnetHTA in July 2023. https://www.eunethta.eu/wp-content/uploads/2023/07/July-13-HTD-meeting-D5.1-and-D5.4.pdf
Preparedness of HTA bodies
While HTA bodies throughout Europe are preparing for the implementation of the HTAR, there are still no details available about the adaptation of national processes to align with the EU-wide JCA. With the finalization of EUnetHTA 21’s deliverables in September 2023 and the outstanding adoption of the procedural and methodological guidelines by the HTACG, HTA bodies may still need to get familiar with the detailed European procedure before formalizing the new structure of the national processes. However, discussions around structural changes and additional evidence requirements on a national level are increasingly taking place. Especially in “first launch” countries like Germany where the AMNOG dossier is currently submitted shortly after EMA marketing authorization when the medicine is placed on the German market, the publication of the JCA report will likely coincide with the current national submission timelines. Delays in the drug launch may thus occur due to additional country-specific analyses (eg, safety endpoints, patient relevance of endpoints) and the validity of methods used (eg, indirect comparisons, surrogate endpoints, single-arm trials) on top of population size and cost analyses. The G-BA is hosting an information event in November to “identify joint opportunities for interlinking the European and German benefit assessment procedures” which will help to inform the national implementation of the EU HTAR in Germany.
In contrast, it is still uncertain how the implementation of the EU HTAR will affect smaller countries in which no established HTA system exists (eg, Cyprus) or where a national procedure has been established only recently (eg, Greece, Bulgaria). These countries should benefit from the centralized European procedure and experience shorter timelines between EMA approval and initiation of the national drug assessment. While an earlier assessment would benefit patients in terms of faster access to drugs, the authorities may face resource and capacity issues caused by the additional work associated with the EU JCA. At the latest information day meeting held in September, HTA bodies from Cyprus, Greece, Bulgaria, and Romania revealed that their preparedness for the EU JCA is low to medium. Implementation of the EU JCA seems to be especially challenging for Cyprus due to the lack of a formalized HTA process and limited resources available. Capacity building and training of patients and clinical experts appeared to be among the most important action items for the countries to prepare for the upcoming EU JCA. Interestingly, authorities from Greece and Bulgaria stated during the information day that in the current systems, products are reimbursed nationally only if these are already reimbursed in at least 5 countries out of a basket of 11 or 17 countries, respectively. With all this in mind, we have to watch closely how the EU JCA will impact national HTA regulations and procedures and, at the same time, the manufacturers’ launch sequence of medicinal products.
Preparedness of clinicians and patients
Patients and clinicians will play a supporting role in the EU HTA during the production of JSCs and JCAs. The EUnetHTA guidance on the involvement of patient and clinical experts during JCA and JSC states that external experts should have a European perspective, but national perspectives should be invited during the national assessments. There are several activities ongoing as part of the EU4Health Work Program to educate external experts on the new processes and HTA in general. Two projects for patients, the European Patients Academy on Therapeutic Innovation (EUPATI) HTA4Patients and the European Capacity Building for Patients (EUCAPA), are currently in their midterm.
The HTA4Patients project is a 3-year training project to enhance the education, training, and information via EUPATI's Open Classroom and Toolbox to support patients with their role in the HTA regulation framework. In addition to already existing information material, an e-learning training course for patients on the specific requirements for the new regulation will be designed.
The EUCAPA project is running from 1 March 2023 until 28 February 2025 and is focusing on building skills and knowledge of patient advocates, preparation of patient organizations to respond to requests for patient experts as part of the joint assessments, and consultations, as well as raising awareness among the wider public about the HTA regulation.
Although these initiatives will be critical to prepare patients and clinicians, clear guidance on how to best identify and contact external stakeholders to enable successful involvement in the JCA/JSC processes still needs to be established by the HTACG.
In conclusion, the publication of key JSC and JCA deliverables provides more information on requirements for biopharma companies. It will be crucial for manufacturers to gauge their internal JCA and JSC awareness and understanding to improve their cross-functional readiness, as well as to build cross-functional capacity and capabilities to execute JCA/JSC and subsequent country processes, and to optimize HTA outcomes. However, not only biopharma companies, but also HTA bodies, patients, and clinicians need to efficiently use the available resources to ensure successful EU HTA processes starting in only 14 months.
Upcoming events related to the EU HTA:
- 17 November 2023: Second HTA Stakeholder Network Meeting
- November 2023: Info day for HTA bodies – Spain, Italy, Malta
- January 2024: Info day for HTA bodies – Austria, Belgium, Ireland, Netherlands, Luxembourg
Key takeaways
- Joint Clinical Assessment (JCA) and Joint Scientific Consultation (JSC) deliverables developed under EUnetHTA 21 still need to be adopted by the Coordination Group on HTA (HTACG); however, refinements are likely necessary.
- Parallel European Medicines Agency (EMA)/health technology assessment (HTA) body Scientific Advice during the interim period has been kicked off in September 2023; advice meetings are likely taking place only in 2024 and meeting spots are limited.
- EUnetHTA 21 has published the JCA submission dossier template which appears to have many similarities with the current national German HTA submission dossier. Evidence requirements in the JCA dossier are comprehensive. To avoid duplication, the evidence presented in the JCA dossier should not be repeated in national HTA submissions; however, local submissions will need to provide additional analyses to fit each country’s specific needs.
- Biopharma companies should initiate internal stakeholder preparation (eg, cross-functional team engagements, upscaling of resources to prepare for parallel EMA/JCA submissions and national processes, statistical analysis) and external stakeholder engagements (eg, participation in joint scientific advice and information events) to ensure a successful JCA process.
- Training of HTA bodies, clinicians, and patients is ongoing and collaboration of all involved stakeholders should be strengthened during the interim period to establish an efficient JCA process from 2025 onwards.
Behring, A. Welche Auswirkungen wird EU HTA auf das AMNOG-Verfahren haben? In: Interdisziplinäre Plattform zur Nutzenbewertung, Issue 17. Springer Medizin Verlag GmbH, Berlin, September 2023.
EPF. EUCAPA. 2022. Accessed 8 September 2023. https://www.eu-patient.eu/projects/ongoing-projects/eucapa/
EUnetHTA. EUnetHTA 21 – Stakeholder meeting. 8 September 2023. Accessed 27 September 2023. https://www.eunethta.eu/wp-content/uploads/2023/09/EUnetHTA-21-Stakeholder-Meeting-08.09.2023-v0.2.pdf
EUnetHTA. D5.1 Submission dossier template – medicinal products. Version 1.0, 31 July 2023. Accessed September 2023. https://www.eunethta.eu/d5-1/
EUPATI. EUPATI HTA4Patients project kicks off – enhancing patients’ knowledge of health technology assessment (HTA) across Europe. 28 February 2023. Accessed 8 September 2023. https://eupati.eu/news/eupati-hta4patients-project-kicks-off-enhancing-patients-knowledge-of-health-technology-assessment-hta-across-europe/
European Commission. Guidance on parallel EMA/HTA body (HTAb) Scientific Advice for the interim period. 3 July 2023. Accessed 27 September 2023. https://www.g-ba.de/downloads/17-98-5512/Guidance%20on%20Parallel%20EMA-HTA%20body%20%28HTAb%29%20Scientific%20Advice%20for%20the%20Interim%20Period.pdf
European Commission. The EU regulation on health technology assessment: what’s in it and why it matters? Accessed 12 October 2023. https://health.ec.europa.eu/system/files/2023-10/hta_20230918_co01_en.pdf
G-BA. Ändert die Implementierung der EU-HTA Verordnung das AMNOG Verfahren? 22 September 2023. Accessed 27 September 2023. https://www.g-ba.de/service/veranstaltungen/amnog-eu-hta-verordnung/
Merész G. HTA Joint Scientific Consultations. How to get most out of Scientific Advice? Presented at: 8th EFSPI Workshop on Regulatory Statistics 2023, 13-14 September 2023.








