How to get earlier market access in the UK: Use the Innovative Medicines Fund
By AmerisourceBergen
By: Jo Watts-James, MBA, and Caroline Hall, PhD
Updated: October 7, 2022



What is the IMF?
The IMF, launched in June 2022, is a pathway for non-cancer drugs to get access to the UK National Health Service (NHS). NHS England promises it “will provide a consistent and transparent managed access process for companies offering promising non-cancer medicines at a responsible price.” It is modelled on the successful Cancer Drug Fund (CDF) framework, which went live July 29, 2016, and provides funding for drugs where there is significant remaining clinical uncertainty, usually drugs with promising phase 1/2 results whilst further data are collected.
Likewise, the IMF is a managed access fund which covers the cost of medicines that show significant clinical promise, whilst evidence generation is ongoing. Any promising new non-cancer medicine can be considered for managed access via the IMF. For any medicine the National Institute for Health and Care Excellence (NICE) recommends with managed access, a managed access agreement (MAA) is put in place between NHS England and the company. MAAs consist of two parts:
- A time-limited Data Collection Agreement (DCA) that outlines the data to be collected as defined by NICE that should address the evidential uncertainties, and
- A patient access scheme (PAS) and/or a commercial access agreement (CAA) to ensure that the MAA offers value to taxpayers during the managed access period.
Under these arrangements, funding could be brought forward by up to 5 months compared with routine commissioning, starting at the point NICE issues a draft positive final guidance.
Which medicines are eligible for the IMF?
The IMF targets the most promising medicines for which there is significant remaining uncertainty around the level of clinical benefit and cost-effectiveness. Medicines will be suitable if they address a high unmet need; provide significant clinical benefits; represent a step-change in medicine for patients and clinicians; and the new evidence to be generated is considered meaningful and could sufficiently reduce uncertainty.
To be included in the IMF, medicines will need to demonstrate their potential to both be cost-effective, as determined by NICE, and deliver value for money by being priced responsibly, as determined by NHS England. The price should reflect both the uncertainty as well as the overall burden imposed on the NHS by any data collection arrangements. Those medicines that show the greatest certainty of clinical benefit and cost-effectiveness are likely to be selected.
How does a drug get onto the IMF?
Companies should engage early with NICE and NHS England & NHS Improvement (NHSE&I) to discuss whether their technology has the potential to be considered for a recommendation with managed access. Effective horizon scanning is essential to allow the NHS to understand the products it will likely be using and give an indication of their possible impact on patients, existing pathways and services, and budgets. Companies are encouraged to make information available at all stages of product development to inform horizon scanning and partners in the wider system. The first points of contact for companies wanting to discuss new products with NICE are via the NICE early engagement opportunities provided through the Office of Market Access (OMA) and, if companies require technical support with evidence generation, the NICE Scientific Advice team is available. Both services have a fee attached. NHSE&I also offers clinical and commercial surgeries later in the development process. The figure below maps out the opportunities for engagement.
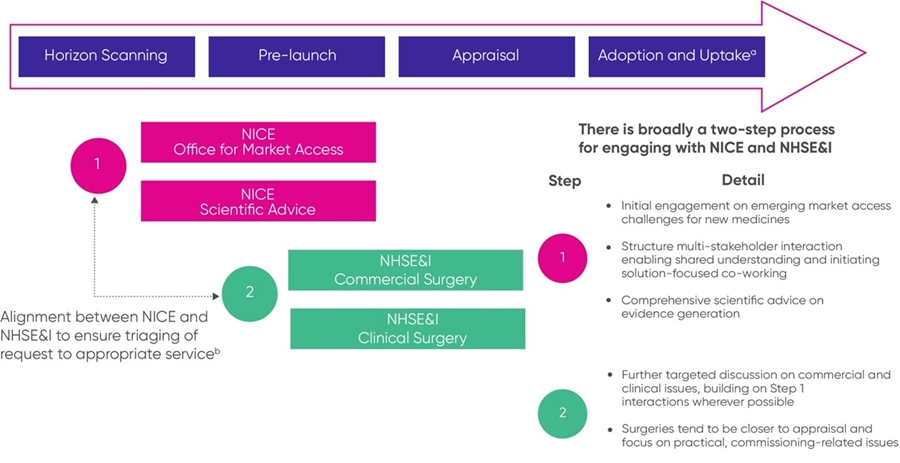
a NHSE&I and NICE both offer advice at later stages of the product life cycle. Requests are triaged appropriately.
b This step acknowledges that confidentiality plays a role in what information can be shared.
How is data collection managed?
Whilst data collection is the responsibility of the developer, NICE supports and facilitates activity at all stages of the managed access process:
- During their evaluation, the NICE Managed Access Team will review the feasibility of data collection and whether it is likely to resolve evidential uncertainties. The NICE Appraisal Committee will identify the uncertainties that prevent the medicine being routinely commissioned in the NHS and the data that could sufficiently resolve these uncertainties.
- Where there is no existing data collection in place, NICE will seek advice from clinicians, patient groups, academics, and data custodians to facilitate the development of potential solutions for companies to consider.
At the end of the data collection period, the data collected, along with any other available and relevant evidence, is submitted to NICE to make the final recommendation on whether the medicine should be routinely available on the NHS.
What costs are involved?
The IMF covers the agreed cost of the medicine whilst the company will be required to cover all costs for data collection, analysis, and updated submission to NICE. It should be noted that the IMF will operate within a fixed budget of £340 million per annum with an expenditure control mechanism (ECM) to ensure that any spend above this is paid back on a proportional basis by all companies receiving funding from the IMF. Agreeing to the ECM will be a condition for all companies benefiting from the IMF and will ensure that the fund does not have to close to new entrants.
Summary
The IMF provides game-changing new drugs that treat disease other than cancer with a route for faster market access. Companies developing products that will offer a step change in treatment should use horizon scanning to engage early with NICE and NHSE&I to start the discussion on whether they are eligible for early market access via the IMF.
Sources
- https://www.england.nhs.uk/medicines-2/innovative-medicines-fund/#:~:text=The%20Innovative%20Medicines%20Fund%20is,%2Dedge%20non%2Dcancer%20treatments
- https://www.england.nhs.uk/medicines-2/innovative-medicines-fund/
- https://www.england.nhs.uk/cancer/cdf/
- https://www.england.nhs.uk/publication/the-innovative-medicines-fund-principles/




