Authorized Generics
Everything You Need to Know
As a healthcare provider in your community, you're a patient's trusted source for prescription medication information.

Now more than ever, consumers have a heightened awareness of the pharmaceutical supply chain, and you may get questions from patients about how prescriptions are sourced and what their options are.

It’s important to share the differences within available prescription options so patients can choose a product that’s safe, effective and fitting of their desired experience and values.

Access a variety of products to enhance the choices available to your patients
Both authorized generic and regular generic drugs are offered within our generics formulary, ensuring access to a variety of options that both meet patient needs and allow you to maximize your rebate.

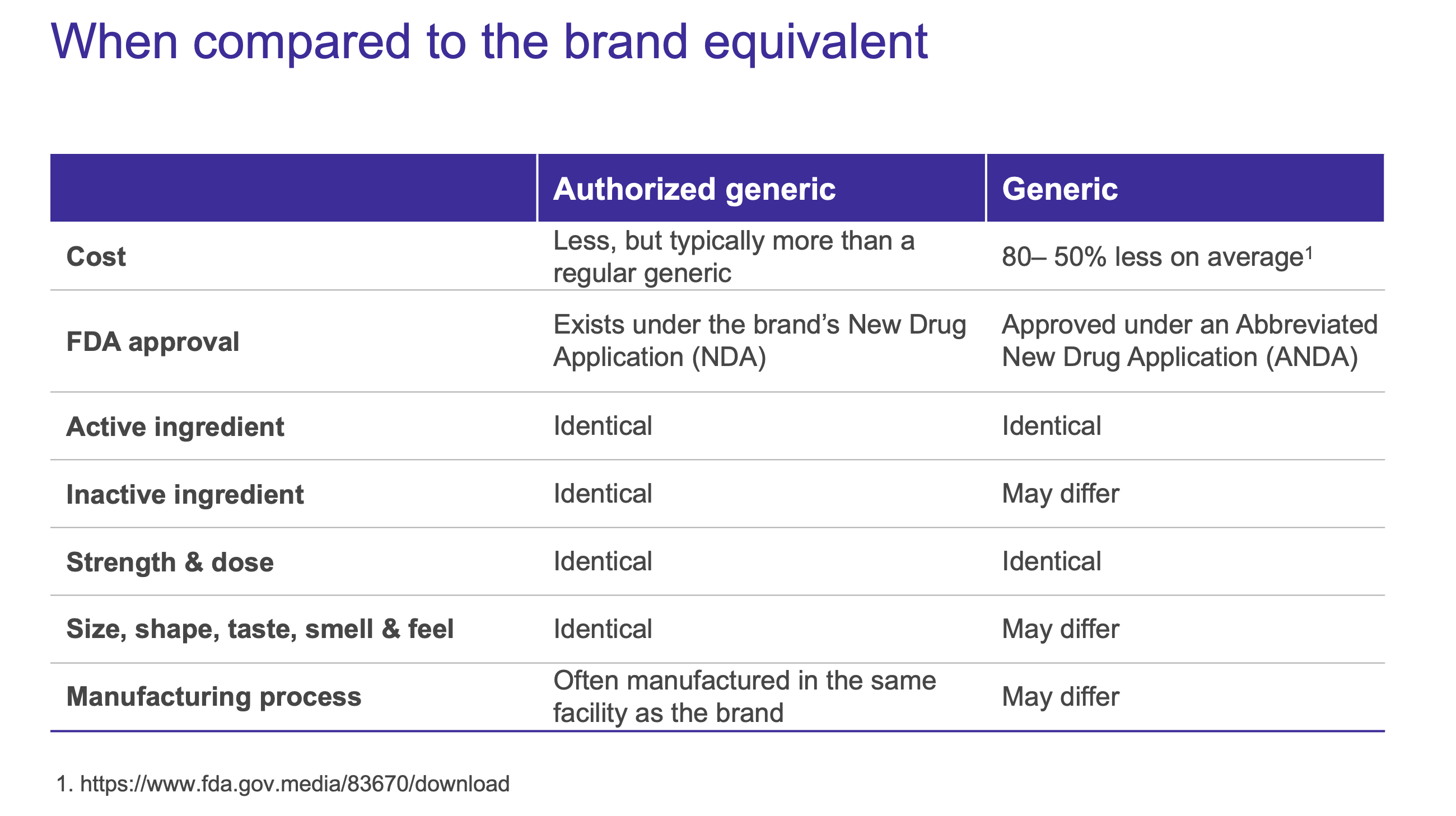
Available Options — What’s the difference?
Generic drugs are generally familiar to patients as a less expensive alternative to its branded counterpart. Though similar in name, authorized generics are a unique classification not to be confused with generic drugs whose approvals are submitted through the Abbreviated New Drug Application (ANDA) process. Authorized generics are developed under the branded drug’s New Drug Application (NDA), making less-expensive options available even before a brand patent expires.